Share this
Transdermal drug delivery systems: all you need to know
by Zakia Moulla on Jan 19, 2024 9:45:02 AM
With an average estimate of one transdermal product receiving FDA approval every 2.2 years, transdermal drug delivery has emerged as a multi-billion-dollar industry. Offering numerous advantages, transdermal drug delivery systems are considered an effective alternative to oral drug delivery and intravenous injections. Continue reading to delve into the products, types, and benefits of transdermal drug delivery systems.
-1.png?width=3024&height=1890&name=Blog%20Article%20Pictures%20(2)-1.png)
1. What are transdermal drug delivery systems?
Commonly referred to as patches, transdermal drug delivery systems (TDDS) are dosage forms developed to deliver a therapeutically effective amount of a drug directly through the patient's skin into the bloodstream. Due to their ease of administration, excellent patient convenience and adherence, TDDS offer an advantageous alternative to oral drug delivery systems like tablets or syrups. They find applications not only in pharmaceuticals but also in the skincare industry, including cosmetics.
Transdermal drug delivery systems are specifically designed for systemic treatments by delivering drugs through the patient’s skin. Offering controlled release and maintaining continuous efficacy of the drug, they are considered an effective drug delivery method. The drug released from TDDS initially permeates the skin through the stratum corneum progressing through the epidermis and dermis without accumulating the active ingredient in the dermal layer. Once the drug reaches the dermal layer, it is released for systemic absorption via the dermal microcirculation.
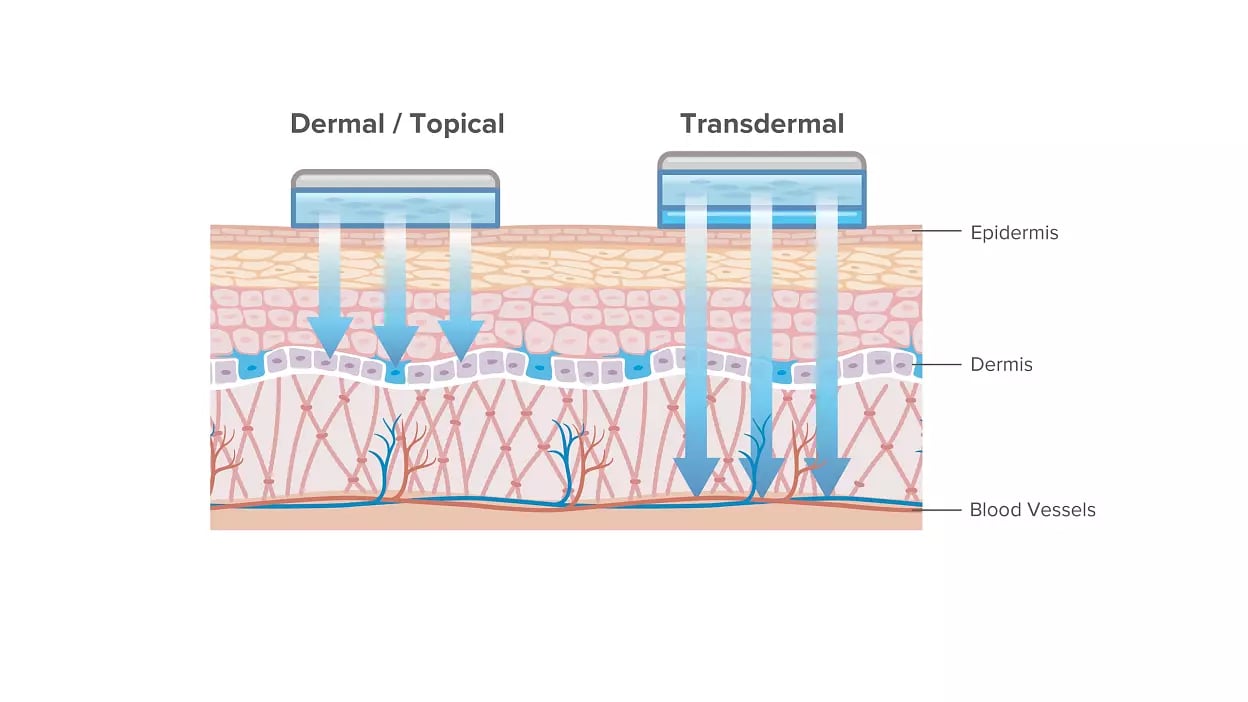
Overall, transdermally applied drugs are safely and conveniently delivered to children and elderly patients, treating a wide range of conditions and diseases. These include moderate to severe menopausal vasomotor symptoms, Alzheimer’s disease, ADHD, major depressive disorder, chronic pain management, and various other conditions.
2. Transdermal drug products:
The world's first transdermal system for the systemic administration of drugs was a three-day scopolamine patch for treating motion sickness, approved in the United States in 1979. Just a generation later, the first blockbuster transdermal product, the nicotine patch, significantly increased the perceived value of transdermal delivery among the healthcare community and the general population. Nowadays, there are numerous transdermal delivery systems for drugs like oestradiol, fentanyl, and testosterone. Additionally, there are combination patches containing multiple drugs for contraception and hormone replacement.
This is a list of Drug Products in some transdermal patches available on the current market:
3. Types of transdermal patches:
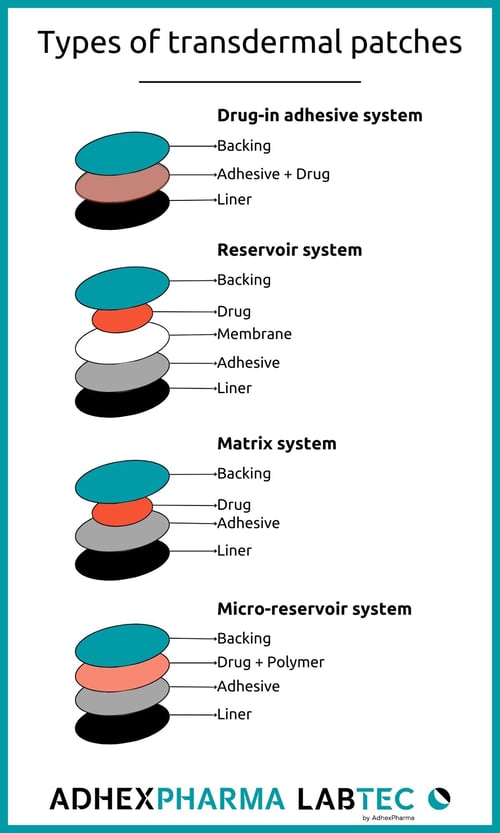
Transdermal patches are generally categorized into four groups based on their manufacturing types: drug-in-adhesive (DIA), reservoir, matrix, and micro-reservoir systems.
1. Drug-in-Adhesive System (DIA):
The patch comprises a layer of adhesive containing the active ingredient. Upon application, the adhesive layer adheres to the skin and gradually releases the drug during the wearing period.
2. Reservoir System:
In patches with a reservoir system, drugs are contained in a separate liquid layer, isolated from the adhesive. When placed on the skin, the drug is released from the liquid layer through a microporous rate-controlling membrane.
3. Matrix System:
The system includes a drug layer with a semi-solid matrix that contains a dispersed drug solution or suspension with a polymeric pad directly in contact with the skin. The adhesive layer in this patch surrounds and partially covers the drug layer.
4. Micro-Reservoir System:
Combining features of both the reservoir and matrix systems, the micro-reservoir system suspends the active ingredient in an aqueous solution of a water-soluble liquid polymer. This mixture is then uniformly dispersed in a lipophilic polymer, creating numerous non-leaching microscopic drug reservoirs.
5. Basic components of transdermal patches:
The essential components of transdermal patches include:
- Drug-containing reservoir or matrix
This component serves as the reservoir for the active drug. The drug may either be dissolved in a liquid reservoir or dispersed within a matrix. It constitutes the primary ingredient responsible for transdermal delivery through the skin, ensuring controlled release and absorption of the API into the body. - Adhesive
The adhesive layer sticks the patch to the skin. It ensures that the patch remains in place during wear and facilitates the transfer of the drug from the patch to the skin. - Liner or protective layer:
A removable liner covers the adhesive layer before use to protect it and maintain its integrity until application. This layer is peeled off before applying the patch to the skin. - Backing
The backing layer provides structural support to the patch and protects the drug-containing layer and adhesive from external factors like moisture and contamination. It also prevents the drug from leaking out of the patch. - Controlled release membrane
In some transdermal patches, a controlled release membrane or rate-controlling membrane may be included. This membrane controls the rate at which the drug is released from the patch into the skin.
6. Transdermal drug delivery advantages:
TDDS has revolutionized the delivery of a wide range of therapeutic agents, particularly in pain management, hormone therapy, and the treatment of cardiovascular and central nervous system disorders. Known for being patient-friendly, they are non-invasive, reducing gastrointestinal side effects and enhancing patient adherence.
This approach eliminates the use of invasive, irritating needles, reducing medical waste and infection risks. Additionally, it bypasses the first-pass effect in the liver, improving bioavailability, efficacy, and translocation. TDDS can be engineered to provide continuous drug delivery over longer intervals (up to several days), reducing administration frequency compared to oral dosage forms.
7. Summary
In conclusion, transdermal patch technology stands out as a valuable drug delivery method, offering substantial advantages over other routes, especially when the Active Pharmaceutical Ingredient (API) is well-suited for transdermal delivery. This technology has made significant contributions to medical practice. For more information about transdermal patches, please feel free to contact us here. At AdhexPharma, we specialize in the development and manufacturing of patches and oral films, providing tailored solutions for your product ideas.
References:
- Jeong, W.Y. et al. (2021) Recent advances in Transdermal Drug Delivery Systems: A Review, Biomaterials research. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8317283/
- Wong, W.F. et al. (2023) Recent advancement of medical patch for Transdermal Drug Delivery, Medicina (Kaunas, Lithuania). Available at: https://ncbi.nlm.nih.gov/pmc/articles/PMC10142343/
- Purushotham, K. and Anie Vijetha, K. (2023) A review on Transdermal Drug Delivery System. Available at: https://gsconlinepress.com/journals/gscbps/sites/default/files/GSCBPS-2023-0053.pdf
- Hanbali, O.A.A. et al. (2019) Transdermal patches: Design and current approaches to Painless Drug Delivery, Acta Pharmaceutica. Available at: https://sciendo.com/article/10.2478/acph-2019-0016
